Management Responsibility and Control in Pharmaceuticals
Management
responsibility is fundamentally no different in the pharmaceutical industry than
any other business. The quote “The
buck stops here” made famous by the sign on the former U.S. President
Harry S. Truman’s
desk applies to anyone who holds the top job in any industry. The top
job is where the ultimate accountability resides for the company strategies and
decisions to achieve the intended
outcomes of the enterprise. For pharmaceuticals, management is individually responsible for ensuring that systems to comply with current good manufacturing practice
(CGMP) regulations are effectively implemented in order to establish and maintain a state of control for drug manufacturing, holding, and
distribution.
CGMPs
and state of control are inextricably connected. Lack of control leading to a
Field Alert and Recall due to the
potential of product being materially affected will be cited as violations of CGMPs.
The converse is also true. A pattern
of CGMP violations observed during a Food and Drug Administration (FDA) inspection will
also be viewed as lack of control, whether or not the product is materially affected. Either way, the
associated product will be deemed adulterated within the meaning of the Federal Food, Drug and Cosmetic Act (FDCA).
The implication of the impact of CGMP noncompliance on the business
is not theoretical. There are ample examples in the pharmaceutical
industry where ineffective implementation of CGMP systems resulted in loss of control that materially affected
product quality, which, in turn, affected inventory
and patient supply. Establishing a Pharmaceutical Quality System (PQS) that
effectively implements the CGMPs is
the means for maintaining a state of control—the fundamental intent of these regulations.
Management
does not assume positions of responsibility with the intent of neglecting CGMP compliance. However, management may not
enter the top position fully equipped to assume responsibility for CGMPs in a practical way. Management may delegate all CGMP matters
to the Quality Department and take a hands-off approach and rely on this function to bring matters
to its attention at their discretion. Such passivity leads to hearing
only the bad news when it is far
too late to contain and resolve the problem in the most cost-effective way with
least risk to public safety.
Likewise,
some Quality Departments may not be adequately equipped to bridge the space between top management and daily
operations with effective structures and processes that enable management to exercise its responsibility
for CGMP oversight. Too often, the default position is to rely upon the outcome of regulatory inspections. But as one might expect,
a good outcome can give a
false sense of security, and a poor outcome can be viewed as the exhaustive
list of problems. As in any area of
the business where risks must be managed, there is no better approach than
having an intentional management
system in place that provides actionable data to know internally where your daily
operation stands at any given moment.
This
chapter provides a model for structuring a practical and effective management
system to fulfill this expectation. But first, it is useful
to understand the background for the FDA requirement for management to exercise its responsibility for oversight and control.
THE REGULATORY BASIS FOR MANAGEMENT RESPONSIBILITY
Historically, the FDA has cited the Supreme
Court decisions of United States
v. Dotterweich (1943)1 and United
States v. Park (1975)2 as
FDCA legal cases
that establish that the manager
of a corpora- tion can be
prosecuted under the Federal FDCA, even if there is no affirmation of wrong
doing of the corporation manager individually.
In
the Dotterweich case, the jury found Dotterweich, the president and general
manager of a drug repackaging
company, guilty on two counts for shipping misbranded drugs in interstate com- merce, and a third for shipping an
adulterated drug. One dissenting judge of the Circuit Court of Appeals reversed the decision on the
grounds that only the corporation was the “person” subject to prosecution, thus protecting the president personally. But the Supreme
Court reversed the decision, thus holding Dotterweich individually
responsible, not just the manufacturer. Justice Frankfurter delivered the opinion of the Court,
“…under § 301 a corporation may commit an offense and all persons
who aid and abet its commission are equally guilty….”
In
the Park case, the chief executive officer was found guilty on all counts
involving food held in a building
accessible to rodents and being exposed to contamination by rodents, resulting
in the adulteration of the food within the meaning of the FDCA. Park’s defense
was that he had an organi- zational
structure responsible for certain functions to handle such matters. However,
evidence from inspections of multiple locations
indicated the same problems and inadequate system for which he had overall responsibility. Chief Justice
Burger delivered the opinion of the Court, “…by reason of his position in the corporation,
responsibility and authority either to prevent in the first instance, or promptly to correct, the violation
complained of, and that he failed to do so…the imposition of this duty, and the scope of the duty,
provide the measure
of culpability…”
More
recently, Public Law 112–144 (July 9, 2012) called the Food and Drug
Administration Safety and Innovation
Act (FDASIA) added to the definition of CGMP in the FDCA (Section 501, 21 USC 351) to explicitly include management oversight of manufacturing to ensure quality.
Section 711 of FDASIA states:
For
the purpose of paragraph (a)(2)(B), the term “current good manufacturing
practice” includes the implementation
of oversight and controls over the manufacturing of drugs to ensure quality,
including managing the risk of and
establishing the safety of raw materials, materials used in the manufacturing of drugs, and finished drug products.
The addition
of oversight and controls to the definition of CGMP has strengthened the FDA position
with specific language for management’s responsibility for oversight and control as a requirement in the Act. The question remains how to practically and operationally to perform this responsibility. The fol- lowing model describes essential elements of a CGMP Management System for oversight and control.
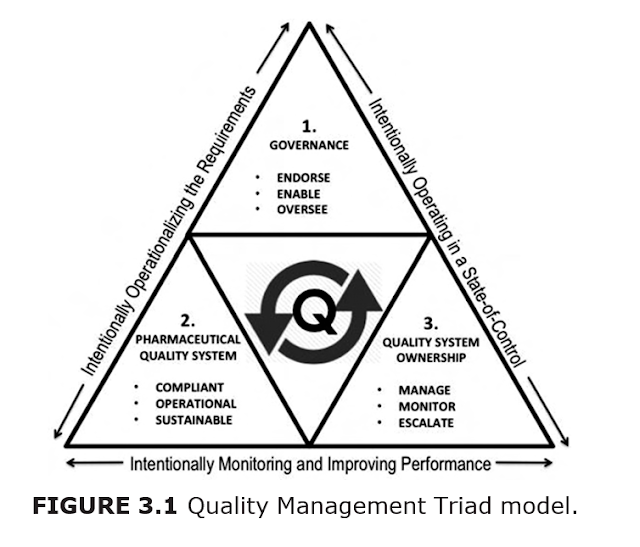
THE QUALITY
MANAGEMENT TRIAD MODEL
Three
elements form the framework of this model for designing and implementing
structures and processes that enable
management to exercise responsibility for oversight and control of CGMP compliance: Governance, the Pharmaceutical
Quality System, and Quality System Ownership.
These elements are interdependent, and the effectiveness of the model depends upon how well they are designed to work together. The
following sections discuss the key attributes of each element of the Quality
Management Triad model (Figure 3.1).
The Governance element
The governance body is comprised of the top leadership where the ultimate direction is given and decisions are made with respect to aligning resources, including those associated with CGMP compliance. Governance is the standard-bearer for what it endorses, enables, and oversees. To the watching organization, what management says, funds, and pays attention to, are the visible expres- sions of their values and the cultural setting for CGMP compliance.
Endorse
The governance body endorses a Quality Policy that declares the importance of CGMP compliance to the business by ensuring a continuous of supply of quality product to its patients. This policy provides the foundation for creating a set of Quality Standards that interprets and applies the CGMP regulations and current industry practice to its operations. Quality Standards are cross-functionally endorsed to demonstrate agreement with the minimum, irreducible requirements. Although Quality
Standards
establish the “What,” there is latitude for designing processes for “How”
requirements are met. Thus, CGMP
processes are opportune targets for continuous improvement. The “How” described in standard operating procedures
(SOP) is open to creative approaches for designing processes as efficiently as possible that also comply with the Quality Standards, and thus CGMP.
Enable
The
governance body enables CGMP compliance by providing the organization structure
and resources to implement and support processes
described in SOPs. While it is reasonable for manage- ment to question
whether a process
is designed as efficiently as possible, it is not reasonable to with- hold resources that affect the ability to
comply with Quality Standard requirements. Governance also establishes and partners with an independent Quality Unit
that has the product knowledge, technical
experience, and CGMP background to perform its unique regulatory
responsibilities. The Quality Unit
has the responsibility to objectively measure and report on the effectiveness
of the PQS, and management has the responsibility for oversight and control.
Oversee
The
governance body establishes the Quality Management Review (QMR) process to
oversee the ongoing performance of
the PQS and to give visibility to problems and risks as a means of under- standing the state of control. The QMR
process is a data-driven and action-oriented process to ensure that unacceptable risks and trends
are quickly identified and verifiably resolved.
The Quality Unit is best positioned to administer
the QMR process to ensure objective reporting, proper risk assessment, and verification that the intended
results of QMR decisions have been achieved.
Normal business processes,
such as planning and budget cycles, and operational goals and performance objectives, serve also to prioritize and focus company
attention where significant change or effort is necessary to improve CGMP compliance.
The
PharmaceuTical
QualiTy SySTem elemenT
The PQS is the interpretation and application of CGMP regulations to the operation. It is an integrated library of documents of increasing specificity from the Quality Policy and Quality Standards down to the SOP, and other executable work instructions. Like a balanced outline, the PQS has an overall structure with each topic taking its position as part of an intentional architecture. SOPs must be fit-for-purpose and remain relevant within a dynamic operational environment. Thus, the PQS is a living system that reflects the requirements, but it also is operational and sustainable the moment its documents become effective.
Compliant
Procedures and the records created by following procedures provide the legal history that affirms that the work was performed in compliance with requirements and that the expected results were obtained. There is no work related to manufacturing, holding, or distributing of drug product that is not described in an approved,
written procedure. There is a traceable path from the results entered
onto a record up through procedures and directly to standards and policies. It cannot be underestimated the importance to CGMP compliance of having clearly written procedures that logically describe the process steps, the persons who perform them, where results are recorded, and who reviews and interprets. Writing simply and clearly, along
with communication and training are special skills
required to establish effective procedures so that CGMP requirements are faithfully translated to those doing the work.
Operational
The
PQS is intended to work in the actual operation as soon as a procedure is
approved and implemented through training.
Process flow diagrams
are a useful tool in the hands of the user groups
to visualize the real-world steps of a process. They facilitate
discovery of the supplier of inputs, customers of outputs, and dependent linkages
to other processes that may need to be developed
or aligned for the overall process
to be effective. When and who makes decisions, as well as the conditions or situations in which the
Quality Unit is notified are also part of SOP content for the daily operation. For the PQS to be operational,
the user groups must actively participate to identify the obstacles
to getting work accomplished and having meaningful procedures.
Sustainable
The PQS must be sustainable by providing a supporting organization structure, adequate number of
skilled personnel, and an operating budget that are balanced with the demands
on the system. Significant operational changes such as acquisitions, facility
repurposing, production volume,
port- folio complexity, and
workforce levels invariably impact the effectiveness of PQS and present a compliance risk if not anticipated and
addressed. Performance metrics are the primary tools to continually monitor the performance of the PQS and to provide
the operational capability to self- detect
and self-correct problems. This capability is essential since continual
performance feedback is fundamental for sustaining performance and operating
in a state of control.
The Quality System
ownership element
The
PQS lives in a dynamic business and regulatory environment. Thus, the PQS must
be continually reevaluated to ensure its purpose is served. The Head of Quality
has functional responsibility for the
overall PQS, and management has the overall responsibility for oversight and
control. But knowledgeable and
skilled Quality System Owners (QSO) at the operational level are needed to take responsibility for ensuring that
their respective processes and procedures are continuously relevant, compliant, and integrated. Each
QSO has the responsibility for managing their part of the PQS,
monitoring performance, and escalating significant issues to management.
Manage
The
QSO is responsible for the resilience of their respective parts of the PQS
amidst the business changes around
them. The QSO has the knowledge and skills to ensure that his/her processes continue
to meet compliance requirements, are relevant to the operation,
fit-for-purpose, and perform effectively
and efficiently. This requires being up to date with CGMP regulations and
developing industry awareness
to remain current
with industry best practices. The QSO is the champion
for their respective processes and leads the
adoption of significant changes, as well as ongoing continuous improvement. The QSO is the subject-matter-expert and the face of their respective PQS processes.
Monitor
Data-driven information in the hands of a responsible and empowered QSO is essential
to the ongoing management of the system and maintaining a state of
control. Each QSO has carefully selected metrics
that provide ongoing and objective feedback about their respective part of the
PQS. There may be key performance
metrics adopted by the company or a site operation. There may also be popular ideas about metrics and what they
should or should not be. There may even be rules of thumb to limit the number of key performance metrics.
However, these should
not be confused with whatever performance metrics are personally
needed by each QSO to have the data necessary to take responsibility for the daily management and problem
detection. In the end, the QSO must be able
to answer two questions at any moment: How well is the system operating? What
problems or potential problems
is the system detecting? The QSO is in the best position
to decide on the performance metrics for the
systems for which they are responsible.
Escalate
The QSO must have directed a path to management to escalate problems
or potential problems
that represent unacceptable or unmanageable risk to the patient, the business, or CGMP compliance. The Quality Management
Review is the regularly occurring
forum where QSOs have the opportunity
to report on the state of their system. But there are instances where the degree of risk must permit
a direct and unfiltered alert to management. Some problems or potential
problems rise to the level where
either the cost of the permanent solution or the cross-functional impact
requires making the case directly and expeditiously to higher levels of management.
The role of Quality
Everyone
owns “CGMP compliance” much like everyone owns safety. Everyone has personal responsibility for having general CGMP
knowledge and awareness, and also having and following specific procedures relevant to their areas of responsibility.
However, the Quality Unit ensures that a strategy, such as this Quality Management Triad model, is in place
procedurally, in use behavior- ally,
and has the capability to objectively measure and report on the state of
control.
CONCLUSION
Management
has the legal responsibility for implementing the CGMP regulations and
overseeing and ensuring the
operational state of control. To operate in a state of control does not mean
perfection. It does mean, however, the capability of a firm to self-detect and
self-correct potential problems. And whenever a problem does emerge, the firm
is capable of taking action to understand the
underlying causes the problem or unacceptable trend, and make decisions
that favorably effect the trend, or that prevent
recurrence of the problem. The model described here provides the framework for developing and implementing a
practical and integrated system for management to have the means to exercise
responsibility and have data-driven knowledge of the state
of control.
REFERENCES
1.
United States v. Dotterweich, 320 U.S. 277 (1943)
2. United States
v. Park, 421 U.S. 658 (1975)
SUGGESTED READINGS
• J. Snyder, “Management Oversight of the Pharmaceutical Quality System: Obstacles and Opportunities.” Journal of GXP Compliance 17 (2), 2013, available at: http://www.ivtnetwork. com/management-oversight.
• J. Snyder, “Management Responsibility for the Quality System: A Practical Understanding for the CEO in FDA-Regulated Industries.” Journal of cGMP Compliance 3 (3), 55–59, 1999.
• J. Snyder, “Good Manufacturing Practices: Steps to Improve Quality,” invited author for chapter in Pharmaceutical Sciences Encyclopedia: Drug Discovery, Development, and Manufacturing. Ed. Shayne Gad. New York, John Wiley & Sons, 2010, Published online doi:10.1002/9780470571224.pse406.
J. Snyder, “Mindful Compliance: Where Knowledge and Regulations Meet.” BioPharm International Supplement, Guide to Good Manufacturing Practices, 26–34, 2004.
Reference: Good Manufacturing Practices for Pharmaceuticals (2020)



.webp)





%20Web%20of%20pharma%20.webp)



