Cleanrooms
Cleanrooms are an essential function in the processing of pharmaceuticals, biologics, and medical devices that are safe and effective. Cleanrooms provide an environment that minimizes sources of potential contaminates such as dust, microbes, particles, and vapors. Cleanrooms are classified by the maximum number of particles allowed per cubic meter. The controlled dispersion of particulates or contaminates by airflow is essentially how a cleanroom maintains a clean environment. ISO 14644-1:1999 defines a cleanroom as a “room in which the concentration of airborne particles is controlled, and which is constructed and used in a manner to minimize the introduction, generation, and retention of particles inside the room, and in which other relevant parameters, e.g. temperature, humidity, and pressure, are controlled as necessary.”
Two types of cleanrooms exist: unidirectional and non-unidirectional. Each type of cleanroom has its unique advantages and disadvantages. Unidirectional cleanrooms are necessary when an ISO class 6 or better cleanroom is required. Non-unidirectional cleanrooms are generally less expensive to construct and rely on a high volume of air to disperse contaminates. Air filtration is essential to maintain acceptable particulate/contaminate counts. Two types of filters can be used: high-efficiency particulate air (HEPA), which are at least 99.97% efficient, and ultra-low penetration air (ULPA), which are at least 99.999% efficient. HEPA filters are used for ISO class 6 to 8 cleanrooms and ULPA filters are used for ISO class 1 to 5 cleanrooms. Cleanrooms are classified by the maximum number of particles allowed per cubic meter (Table 28.1 through Table 28.4). Cleanrooms rely on the dispersion of particulates/contaminates by airflow (Table 28.5).
When it is necessary to control the environment, specifications for parameters such as temperature, humidity, colony-forming units (CFUs), and particulates per cubic foot should be established. No United States Food and Drug Administration (FDA) guidances for these parameters presently exist for environmentally controlled areas such as cleanrooms.
A review of 21 CFR 210, 211, and 820 yields no direct requirements for the use or classifications of controlled environments or cleanrooms. These parts of 21 CFR do have references requiring the need for environmental and contamination controls where a lack of control could reasonably be expected to have an adverse effect on product quality. However, FDA has issued a guidance for industry, Sterile Drug Products Produced by Aseptic Processing—Current Good Manufacturing Practice. This guidance document recommends the use of an ISO class 5 cleanroom (European Union [EU] Grade A) in areas deemed to be critical. A critical area is defined as “one in which the sterilized drug product, containers, and closures are exposed to environmental conditions that must be designed to maintain product sterility.” FDA recommends that areas directly supporting aseptic processing activities be at a minimum ISO class 7 while in operation. Consistent with Volume 4 EU Guidelines to Good Manufacturing Practice Medicinal Products for Human and Veterinary Use, Annex 1 Manufacture of Sterile Medicinal Products, it is recommended that microbiological contamination (bioburden) counts remain at < 1 at all times.
SPECIFIC ISO SECTIONS RELATED TO
ISO 14644-2:2000
CLEANROOMS
Specifications for testing and monitoring to prove continued compliance with ISO 14644-1 provide guidance and direction for testing requirements to ensure the proper operation and compliance for cleanrooms. According to ISO 14644-1:1999, cleanrooms may be classified in one of three states:
• As-built. Condition where the installation is complete, with all services connected and functioning, but with no production equipment, materials, or personnel present
• At-rest. Condition where the installation is complete, with equipment installed and operating in a manner agreed on by the customer and supplier, but with no personnel present
• Operational. Condition where the installation is functioning in the specified manner, with the specified number of personnel present and working in the manner agreed on
The minimum number of sampling points is derived from the following equation:
Where: NL = The minimum number of sampling locations (rounded up to a whole number)
A = The area of the cleanroom or clean zone in square meters
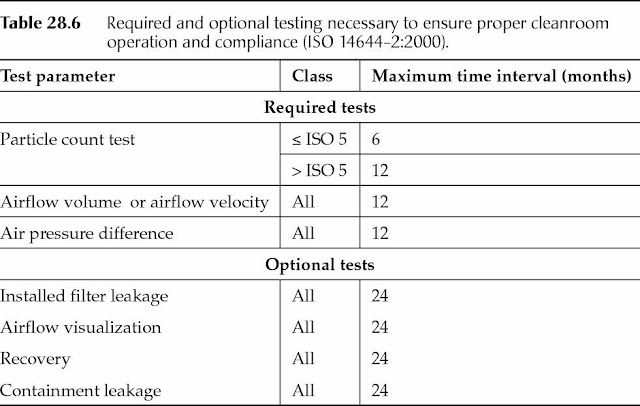
Table 28.6, derived from ISO 14644-2:2000, lists the required and optional testing necessary to ensure proper cleanroom operation and continued compliance.
ISO 14644-1:1999
This part of ISO 14644 (Cleanrooms and associated controlled environments—Part 1: Classification of air cleanliness) covers the classification of air cleanliness in cleanrooms and associated controlled environments exclusively in terms of concentration of airborne particles. Only particle populations having cumulative distributions based on threshold (lower limit) sizes ranging from 0.1 μm to 5 μm are considered for classification purposes. This part does not provide for classification of particle populations that are outside of the specified particle size range, 0.1 μm to 5 μm. Concentrations of ultrafine particles (particles < 0.1 μm) and macroparticles (particles
> 5 μm) may be used to quantify these populations in terms of ultrafine descriptors and macroparticle descriptors, respectively. This part of ISO 14644 can not be used to characterize the physical, chemical, radiological, or viable nature of airborne particles. ISO 14644-2:2000
This part of ISO 14644 (Cleanrooms and associated controlled environments—Part 2: Specifications for testing and monitoring to prove continued compliance with ISO 14644-1) specifies requirements for periodic testing of a cleanroom or clean zone to prove its continued compliance with ISO 14644-1 for the designated classification of airborne particulate cleanliness. These requirements invoke the test described in ISO 14644-1 for classification of a cleanroom or clean zone. Additional tests are also specified to be carried out in accordance with the requirements of this part of ISO 14644. Optional tests, to be applied at the user’s discretion, are also identified. This part of ISO 14644 specifies requirements for monitoring of a cleanroom or clean zone (hereafter referred to as an installation) to provide evidence of its continued compliance with ISO 14644-1 for the designated classification of airborne particulate cleanliness.
ISO 14644-3:2005
ISO 14644-3:2005 (Cleanrooms and associated controlled environments—Part 3: Test methods) specifies test methods for designated classification of airborne particulate cleanliness and for characterizing the performance of cleanrooms and clean zones. Performance tests are specified for two types of cleanrooms and clean zones: those with unidirectional flow and those with non-unidirectional flow, in three possible occupancy states: as-built, at-rest, and operational. ISO 14644-3:2005 is not applicable to the measurement of products or of processes in cleanrooms or separative devices.
ISO 14644-4:2001
This part of ISO 14644 (Cleanrooms and associated controlled environments—Part 4: Design, construction and start-up) specifies requirements for the design and construction of cleanroom installations but does not prescribe specific technological or contractual means to meet these requirements. It is intended for use by purchasers, suppliers, and designers of cleanroom installations and provides a checklist of important parameters of performance. Construction guidance is provided, including requirements for start-up and qualification. Basic elements of design and construction needed to ensure continued satisfactory operation are identified through the consideration of relevant aspects of operation and maintenance.
Application of this part of ISO 14644 is restricted in that user requirements are represented by the purchaser or specifier; specific processes to be accommodated in the cleanroom installation are not specified; fire and safety regulations are not considered specifically, so the appropriate national and local requirements should be respected; and process media and utility services are only considered with respect to their routing between and in the different zones of cleanliness. Regarding initial operation and maintenance, only cleanroom construction–specific requirements are considered.
ISO 14644-5:2004
ISO 14644-5:2004 (Cleanrooms and associated controlled environments—Part 5: Operations) specifies basic requirements for cleanroom operations. Aspects of safety that have no direct bearing on contamination control are not considered in this part of ISO 14644, so national and local safety regulations must be observed. This document considers all classes of cleanrooms used to produce all types of products. Therefore, it is broad in application and does not address specific requirements for individual industries. Methods and programs for routine monitoring within cleanrooms are not covered in detail in this part of ISO 14644, but reference should be made to ISO 14644- 2 and ISO 14644-3 for monitoring particles, and ISO 14698-1 and ISO 14698-2 for monitoring microorganisms.
ISO 14644-6:2007
ISO 14644-6:2007 (Cleanrooms and associated controlled environments—Part 6: Vocabulary) establishes a vocabulary of terms and definitions related to cleanrooms and associated controlled environments.
ISO 14644-7:2004
ISO 14644-7:2004 (Cleanrooms and associated controlled environments—Part 7: Separative devices [clean air hoods, gloveboxes, isolators and mini-environments]) specifies the minimum requirements for the design, construction, installation, test, and approval of separative devices, in those respects where they differ from cleanrooms as described in ISO 14644-4 and 14644-5. The application of ISO 14644-7:2004 takes into account several limitations: user requirements are as agreed by customer and supplier, application-specific require addressed, specific processes to be accommodated in the separative-device installation are not specified, fire, safety, and other regulatory matters are not considered specifically, and where appropriate, national and local regulations apply.
ISO 14644-8:2013
ISO 14644-8:2013 (Cleanrooms and associated controlled environments—Part 8: Classification of air cleanliness by chemical concentration [ACC]) establishes the classification of air chemical cleanliness (ACC) in cleanrooms and associated controlled environments in terms of airborne concentrations of specific chemical substances (individual, group, or category) and provides a protocol to include test methods, analysis, and time-weighted factors within the specification for classification. ISO 14644-8:2013 currently considers only concentrations of air chemical contaminants between 100 and 10 to 12 g/m3 under cleanroom operational conditions. ISO 14644- 8:2013 is not relevant for application in those industries, processes, or productions where the presence of airborne chemical substances is not considered a risk to the product or process. It is not the intention of ISO 14644-8:2013 to describe the nature of air chemical contaminants. ISO 14644-8:2013 does not give a classification of surface chemical contamination.
ISO 14644-9:2012
ISO 14644-9:2012 (Cleanrooms and associated controlled environments—Part 9: Classification of surface cleanliness by particle concentration) establishes the classification of cleanliness levels on solid surfaces by particle concentration in cleanrooms and associated controlled environment applications. Recommendations on testing and measuring methods, as well as information about surface characteristics, are also given. ISO 14644-9:2012 applies to all solid surfaces in cleanrooms and associated controlled environments such as walls, ceilings, floors, working environments, tools, equipment, and products. The surface particle cleanliness (SPC) classification is limited to particles between 0.05 µm and 500 µm. Several issues are not considered, including requirements for the cleanliness and suitability of surfaces for specific processes, procedures for the cleaning of surfaces, material characteristics, references to interactive bonding forces or generation processes that are usually time dependent and process dependent, selection and use of statistical methods for classification and testing, and other characteristics of particles, such as electrostatic charge, ionic charges, and microbiological state.
ISO 14698-1:2003
ISO 14698-1:2003 (Cleanrooms and associated controlled environments— Biocontamination control—Part 1: General principles and methods) establishes the principles and basic methodology of a biocontanmination control formal system) for assessing and controlling Biocontamination when cleanroom technology is applied for that purpose. It specifies the methods required for monitoring risk zones in a consistent way and for applying control measures appropriate to the degree of risk involved. In zones where risk is low, it can be used for information.
ISO 14698-2:2003
ISO 14698-2:2003 (Cleanrooms and associated controlled environments— Biocontamination control—Part 2: Evaluation and interpretation of biocontamination data) gives guidance on methods for the evaluation of microbiological data and the estimation of results obtained from sampling for viable particles in risk zones for biocontamination control. It should be used, where appropriate, in conjunction with ISO 14698-1.







.webp)




%20Web%20of%20pharma%20.webp)

%20webofpharma.JPG)


