This post contains Rosuvastatin Calcium USP Monograph USP 44 - NF39, Definition, Identification, Assay, Impurities, Specific Tests, Additional Requirements.
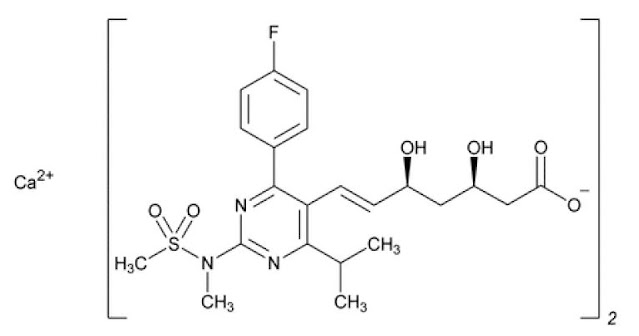
Ca(C22H27FN3O6S)2
6-Heptenoic acid, 7-[4-(4-fluorophenyl)-6-(1-methylethyl)- 2-[methyl methylsulfonyl)amino]-5-pyrimidinyl]- 3,5-dihydroxy-, calcium salt (2:1), (3R,5S,6E); [S-[R*,S*-(E)]]-7-[4-(4-Fluorophenyl)-6-(1-methylethyl)- 2-[methyl(methylsulfonyl)amino]-5-pyrimidinyl]-3,5-dihydroxy-6-heptenoic acid, calcium salt (2:1); Calcium (3R,5S,E)-7-(4-(4-fluorophenyl)-6-isopropyl-2-(N-methylmethylsulfonamido)pyrimidin-5-yl)-3,5-dihydroxyhept-6-enoate salt (1:2) [147098-20-2].
DEFINITION
Rosuvastatin Calcium contains NLT 97.0% and NMT 103.0% of rosuvastatin calcium [Ca(C22H27FN3O6S)2], calculated on the anhydrous and solvent-free basis.
IDENTIFICATION
Change to read:
• A. ▲SPECTROSCOPIC IDENTIFICATION TESTS 97.0%, Infrared Spectroscopy: 197A OR 197K▲ (CN 1-May-2020) or (197A)
• B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the test for Enantiomeric Purity.
• C. IDENTIFICATION TESTS—GENERAL (191), Chemical Identification Tests, Calcium
Sample solution: 8 mg/mL of Rosuvastatin Calcium in a mixture of methanol and water (1:1)
Acceptance criteria: Meets the requirements
ASSAY
PROCEDURE
Protect all solutions containing rosuvastatin calcium and its related compounds from light.
Solution A: Acetonitrile, 1% (v/v) aqueous trifluoroacetic acid, and water (290:10:700)
Solution B: Acetonitrile, 1% (v/v) aqueous trifluoroacetic acid, and water (750:10:240)
Mobile phase: See Table 1.
|
Time (min) |
Solution A (%) |
Solution B (%) |
|
0 |
100 |
0 |
|
30 |
100 |
0 |
|
50 |
60 |
40 |
|
60 |
0 |
100 |
|
70 |
0 |
100 |
|
71 |
100 |
0 |
|
80 |
100 |
0 |
Diluent: Acetonitrile and water (25:75)
System suitability solution A: Dissolve 10 mg of Rosuvastatin Calcium in 10 mL of 1% trifluoroacetic acid in ethyl acetate. Heat at 40° for 1 h. Cool, and transfer to a separatory funnel. Add 2 mL of 1 M sodium hydroxide, and shake for 30 s. Allow the layers to separate, and discard the lower aqueous layer. Add 2 mL of water, and shake for 10 s. Allow the layers to separate, and discard the aqueous layer. Transfer 2 mL of the retained organic layer to a 50-mL standard flask, add 12 mL of acetonitrile, and dilute with water to volume. This solution contains predominantly rosuvastatin lactone.
System suitability solution B: Dissolve 10 mg of Rosuvastatin Calcium in a 50-mL volumetric flask in 10 mL of 1% trifluoroacetic acid in acetonitrile. Stopper, and heat at 40° for 1 h. Cool, add 20 mL of water, and neutralize with 1 M sodium hydroxide to a pH of 6–8. Dilute with water to volume. This solution contains predominantly rosuvastatin diastereomers.
System suitability solution C: 0.25 mg/mL each of USP Rosuvastatin Related Compound A RS and USP Rosuvastatin Related Compound B RS in a mixture of acetonitrile and water (1:1)
System suitability solution D: 0.04 mg/mL of USP
Rosuvastatin Related Compound C RS in a mixture of acetonitrile and water (1:1)
System suitability solution E: Heat 250 mg of Rosuvastatin Calcium at 50° for 7 days in suitable glassware with a porous cover. Dissolve 50 mg of the heated rosuvastatin calcium in 11 mL of acetonitrile in a 50-mL standard flask. Add 5 mL of System suitability solution A, 3 mL of System suitability solution B, 1 mL of System suitability solution C, and 1 mL of System suitability solution D. Dilute with water to volume.
Standard solution: 0.7 mg/mL of USP Rosuvastatin
Calcium RS in Diluent
Sample solution: 0.7 mg/mL of Rosuvastatin Calcium in
Diluent
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 242 nm
Column: 3.0-mm × 15-cm; 3-µm packing L1 Column temperature: 40°
Flow rate: 0.75 mL/min
Injection volume: 10 µL
System suitability
Samples: System suitability solution E and Standard solution
[NOTE—See Table 2 for relative retention times.] Suitability requirements
Resolution: NLT 2.0 between the rosuvastatin and rosuvastatin diastereomer peaks, System suitability solution E
Tailing factor: NMT 1.5 for the rosuvastatin peak,
Standard solution
Analysis
Samples: Standard solution and Sample solution Calculate the percentage of rosuvastatin calcium
[Ca(C22H27FN3O6S)2] in the portion of Rosuvastatin Calcium taken:
Result = (rU / rS) × (CS / CU) × 100
rU = peak response of rosuvastatin from the Sample solution
rS = peak response of rosuvastatin from the Standard solution
cS = concentration of USP Rosuvastatin Calcium RS in the Standard solution
(mg/mL)
cU = concentration of Rosuvastatin Calcium in the Sample solution (mg/mL)
Acceptance criteria: 97.0%–103.0% on the anhydrous and solvent-free basis
IMPURITIES
ORGANIC IMPURITIES
Protect all solutions containing rosuvastatin calcium and its related compounds from light.
Mobile phase, Diluent, System suitability solution E,
Sample solution, Chromatographic system, and System suitability: Proceed as directed in the Assay.
Standard solution: 0.0014 mg/mL of USP Rosuvastatin
Calcium RS in Diluent
Analysis
Samples: Sample solution and Standard solution Calculate the percentage of each impurity in the portion of
Rosuvastatin Calcium taken:
Result = (rU / rS) × (CS / CU) (1/F) × 100
|
Name |
Relative
Retention Time |
Relative
Response Factor |
Acceptance Criteria, NMT (%) |
|
Rosuvastatin related
compound A |
0.9 |
1.00 |
0.2 |
|
Rosuvastatin |
1.0 |
1.00 |
— |
|
Rosuvastatin diastereomersa |
1.1 |
1.00 |
0.5 |
|
Rosuvastatin ketoneb |
1.5 |
0.71 |
0.8 |
|
Rosuvastatin lactonec |
1.7 |
1.00 |
0.15 |
|
Rosuvastatin related
compound Bd |
2.2 |
1.00 |
— |
|
Rosuvastatin dehydro analoge |
1.8 |
1.00 |
0.15 |
|
Rosuvastatin related
compound Cd |
2.6 |
1.00 |
— |
|
Any unspecified impurity |
— |
1.00 |
0.10 |
|
Total impurities |
— |
— |
1.5 |
Result = (rU / rS) × (CS / CU) × 100
rU = peak response of rosuvastatin enantiomer from the Sample solution
rS = peak response of rosuvastatin from the Standard solution
cS = concentration of USP Rosuvastatin Calcium RS in the Standard solution
(mg/mL)
cU = concentration of Rosuvastatin Calcium in the Sample solution (mg/mL)
Acceptance criteria: NMT 0.15%
• LIMIT OF CHLORIDE
Sample: 150 mg of Rosuvastatin Calcium
Titrimetric system
(See Titrimetry á541ñ.)
Mode: Direct titration
Titrant: 0.01 M silver nitrate
Endpoint detection: Potentiometric
Blank: 60 mL of water and 5 mL of 10% (v/v) aqueous
nitric acid
Analysis: Dissolve the Sample in 60 mL of water, and add
5 mL of 10% (v/v) aqueous nitric acid. Titrate with Titrant.
Calculate the percentage of chloride in the portion of
Rosuvastatin Calcium taken:
Result = {[(VS − VB) × M × F]/W} × 100
VS = Titrant volume consumed by the Sample (mL)
VB = Titrant volume consumed by the Blank (mL)
M = actual molarity of the Titrant (mmol/mL)
F = equivalency factor, 35.45 mg/mmol
W = Sample weight (mg)
Acceptance criteria: NMT 0.2%
SPECIFIC TESTS
WATER DETERMINATION (921), Method I, Method I, or Method Ic: NMT 6%
ADDITIONAL REQUIREMENTS
• PACKAGING AND STORAGE: Preserve in well-closed containers, protected from light. Store at controlled room temperature.
• USP REFERENCE STANDARDS
USP Rosuvastatin Calcium RS
USP Rosuvastatin Enantiomer RS
USP Rosuvastatin Enantiomer RS
Calcium (3S,5R,E)-7-[4-(4-fluorophenyl)-6-isopropyl-2-
(N-methylmethylsulfonamido)pyrimidin-5-yl]-
3,5-dihydroxyhept-6-enoate salt (1:2).
Ca(C22H27FN3O6S)2 1001.14
USP Rosuvastatin Related Compound A RS
Calcium (3R,5S,E)-7-{4-(4-fluorophenyl)-2-[(2-hydroxy-
N,2-dimethylpropyl)sulfonamide]-
6-isopropylpyrimidin-5-yl}-3,5-dihydroxyhept-6-enoate
salt (1:2).
Ca(C25H33FN3O7S)2 1117.30
USP Rosuvastatin Related Compound B RS
Calcium (3R,5S,E)-7-(4-(4-fluorophenyl)-2-{2-[4-(4-
fluorophenyl)-6-isopropyl-2-(Nmethylmethylsulfonamido)
pyrimidin-5-yl]-2-hydroxy-
N-methylethylsulfonamido}-6-isopropylpyrimidin-5-yl)-
3,5-dihydroxyhept-6-enoate salt (1:2).
Ca(C38H45F2N6O9S2)2 1703.93
USP Rosuvastatin Related Compound C RS
tert-Butyl 2-[(4R,6S)-6-{(E)-2-[4-(4-fluorophenyl)-6- isopropyl-2-(N-methylmethylsulfonamido)pyrimidin-5- yl]vinyl}-2,2-dimethyl-1,3-dioxan-4-yl]acetate. C29H40FN3O6S 577.71



.webp)





%20Web%20of%20pharma%20.webp)


.webp)
