Stability testing is an integral part of drug development. It is a critical process because on one hand a lot of resources is required to carry out this procedure.
Matrixing and Bracketing
Stability testing is an integral part of drug development. It is a critical process because on one hand a lot of resources is required to carry out this procedure. While on the other side, it is a time consuming process because stability study comprising of one year of data is minimally required for getting the license of a new drug. Hence, it is the need of the hour to design stability protocols in such a manner that they not only satisfy the regulatory authorities but also lessen the consumption of resources and time. Today, Matrixing and bracketing are the two widely adapted and accepted procedures for conducting the stability tests. Their advantages are as follows,
I. Lowered the number of samples of product under consideration for
stability testing
ii. No loss of quality of data
iii. Shelf life of the product remains unchanged
The samples for stability testing should be manufactured, labelled and stored. This step is mandatory to lessen the number of samples taken for stability study along with the reduction in sample production and management cost because storage facilities for sample are quite expensive.
Bracketing
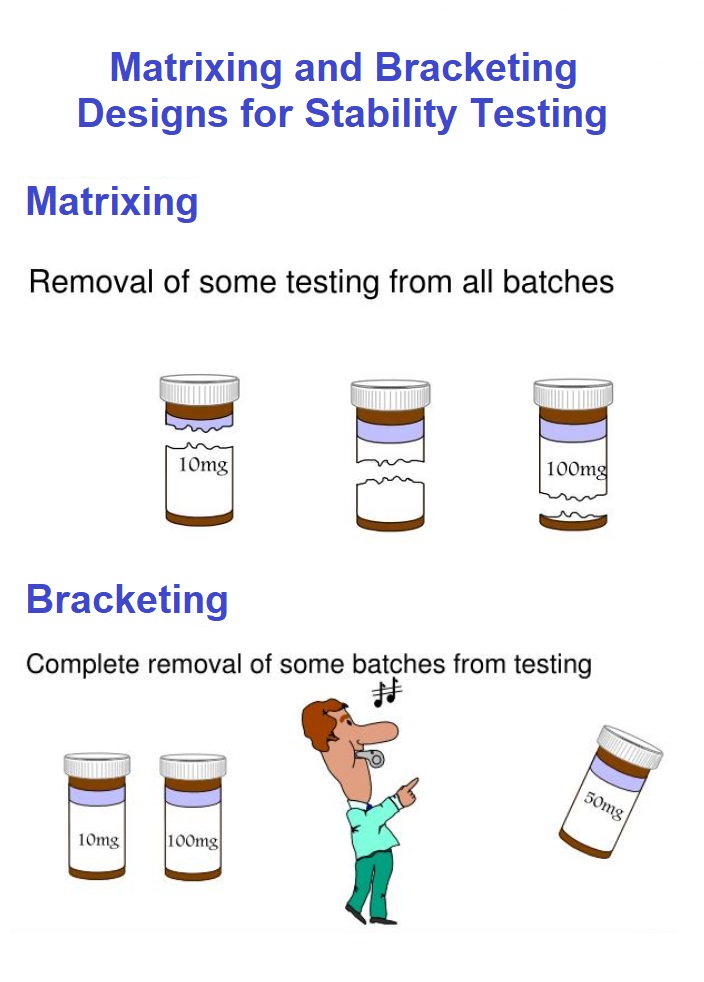
“Bracketing is a stability study design by which only those samples are studied during all the time points as a complete design which have extreme design factors like package size and strength”. It is considered that the stability of extremes represents the stability data of intermediate level. For instance, a tablet having strength as 2mg, 4mg and 6mg is taken for stability testing. So, owing to above mentioned fact, it is possible to overlook the testing of 4mg tablet. But this method cannot be considered as suitable if it cannot demonstrate that the factors for stability testing are extreme. Also, it is applicable in such situations where the multiple identical strengths of a drug are required to be considered for undergoing stability testing. For example,
- Capsules made up of same blend of powders but differ in terms of strength and plug sizes.
- Tablets made up of same granules but differ in strength
- Oral liquid dosage forms having variation with respect to strength and minor excipients like flavoring agents and coloring agents.
In other words, bracketing is applicable to those stability studies wherein multiple strengths are required to be undergone testing with a little change in the quantity of active and inactive ingredients. Thus, such scenarios mainly involve the comparative stability analysis of different strengths of development or clinical batches. This methodology is also applicable to the same container closure system with variable filling amount or container size. Contrary to it, if both the filling amount and container size varies, then the smallest and largest containers doesn’t represent the extremes. As a result, extremes must be selected by carefully examining all the possible characters of the container that may impact the stability. These particular characteristics mainly involve the surface area to volume ratio, thickness of container’s wall, rate of oxygen permeation per dosage unit. Moreover, prior to the application of bracketing, its impact on shelf life of the product must be evaluated with great care.
Matrixing
“The design in which a selected subset of sum of number of all possible samples is tested by considering all possible factors at a particular time interval is known as Matrixing”. Afterwards, another sample can be tested on other time interval. This technique assumes that the result of stability testing of subset under study represents the results of stability testing of all the samples at a specific time interval. This is the reason which makes the stability testing of each batch at a specific time interval unnecessary. However, when the stability of a drug is effected by the secondary packaging container, then Matrixing must be employed across the packaging system. It is further noticed that a separate matrix design must be employed for each storage condition. Also, it is applicable in such situations where the multiple identical strengths of a drug are required to be considered for undergoing stability testing. For example,
- Capsules made up of same blend of powders but differ in terms of strength and plug sizes.
- Tablets made up of same granules but differ in strength
- Oral liquid dosage forms having variation with respect to strength and minor excipients like flavoring agents and coloring agents.
It is recommended to balance the matrix design in order to test each combination in the similar manner at the starting as well as finishing point. But this obligation cannot be achieved in case of matrixed time point. Moreover, generally, this design is suitable when the supporting data predicts the stability of the product. Due to lack of already published data, a matrix design based on factors other than time intervals usually gives less précised evaluation in terms of shelf life.
Author:
Sadia Iftikhar
Department of Pharmacy Practice, Akhtar Saeed College of Pharmaceutical Sciences, Lahore, Punjab, Pakistan



.webp)





%20Web%20of%20pharma%20.webp)


.webp)
