Standard Operating Procedure of Aseptic, Terminal Sterilized Process, Area Grading & Cleaning Procedure in Pharma is describe in this post.
{getToc} $title={Table of Contents}
1. PURPOSE
1.1 To describe the method for aseptic processing, terminal sterilization of Injectable, area classification and its cleaning procedure
2. SCOPE
2.1 The procedure is applicable to sterile section.
3. RESPONSIBILITY
3.1 Operator injectable section is responsible to follow the procedure.
3.2 Production Pharmacist is responsible to
3.3 Production Manager is responsible to implement the procedure.
3.4 Q. A. Officer is responsible to verify the procedure.
4. MATERIAL & EQUIPMENT
4.1 Isopropyl Alcohol
4.2 Dettol
4.3 Proceine 40
4.4 Q’BAC
4.5 Lint free duster
4.6 Mop
5. PROCEDURE
5.1 DEFINITIONS:
5.1.1 CLEAN ROOM
a. A room in which the concentration of airborne particles is controlled, and which is constructed and used in a manner to minimize the introduction, generation, and retention of particles inside the room and in which other relevant parameters, e.g. temperature, humidity, and pressure, are controlled as necessary.
5.1.2 CLEAN ZONE
a. Dedicated space in which the concentration of airborne particles is controlled, and which is constructed and used in a manner to minimize the introduction, generation, and retention of particles inside the zone, and in which other relevant parameters, e.g. temperature, humidity, and pressure, are controlled as necessary.
b. The zone may be open or enclosed and may or may not be located within a clean room.
5.1.3 AS BUILT
a. Condition where the installation is complete with all services connected and functioning but with no production equipment, materials, or personnel present
5.1.4 AT REST
a. Condition where the installation is complete with equipment installed and operation in a manner agree upon by the customer and supplier, but with no personnel present
5.1.5 OPERATIONAL
a. Condition where the installation is functioning in the specified manner, with the specified number of personnel and working in the manner agreed upon
5.1.6 ACTION LEVEL
a. Level set by the user in the context of controlled environments, which, when exceeded, requires immediate intervention, including the investigation of cause, and corrective action.
b. Established microbial or particulate monitoring results requiring immediate follow-up and corrective action.
5.1.7 ALERT LEVEL
a. Level set by the user in the context of controlled environments, giving early warning of a drift from normal conditions, which, when exceeded, should result in increased attention to the process
5.1.8 AIRLOCK
a. Room with interlocked doors designed to maintain pressure control between adjacent rooms of different cleanliness class.
5.1.9 ASEPTIC PROCESSING
a. Handling of sterile product, containers and/or devices in a controlled environment, in which the air supply, materials, equipment and personnel are regulated to maintain sterility
5.1.10 ASEPTIC PROCESSING AREA
a. Facilities for aseptic processing, consisting of several zones
5.1.11 BIO-BURDEN
a. population of viable microorganisms on or in product and/or sterile barrier system
5.1.12 BIO-DECONTAMINATION
a. Removal of microbiological contamination or its reduction to an acceptable level
5.1.13 CLEANING
a. Removal of contamination from an item to the extent necessary for further processing or for intended use.
5.1.14 CORRECTION
a. Action to eliminate a detected nonconformity.
5.1.15 CORRECTIVE ACTION
a. Action to eliminate the cause of a detected nonconformity or other undesirable situation.
5.1.16 CRITICAL PROCESSING ZONE
a. Location within the aseptic processing area in which product and critical surfaces are exposed to the environment.
5.1.17 DE PYROGENATATION
a. Validated process designed to remove or deactivate endotoxins.
5.1.18 ENDOTOXIN
a. Lipopolysaccharide component of the cell wall of Gram-negative bacteria which is heat stable and elicits a variety of inflammatory responses in animals and humans.
5.1.19 PREVENTIVE ACTION
a. action to eliminate the cause of a potential nonconformity or other undesirable potential situation
5.1.20 STERILE
a. Free from viable microorganisms.
5.1.21 STERILIZATION
a. validated process used to render a product free from viable microorganisms
5.1.22 TERMINAL STERILIZATION
a. Process whereby product is sterilized within its sterile barrier system.
5.2 Classification Number: 2.08
5.3 Categories of sterile products
5.4 Aseptic Process
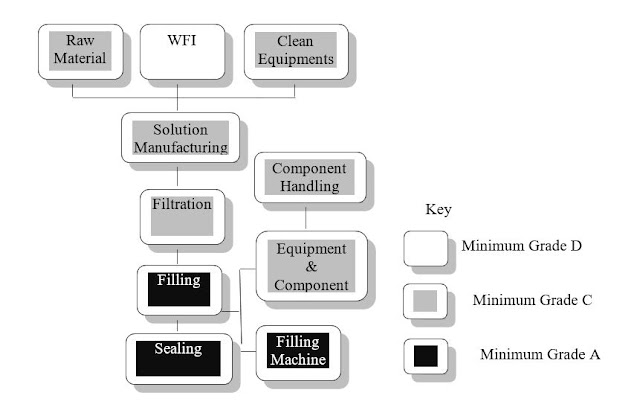
5.5 Terminally Sterilization Process
a. A simple Process Flow for Terminally Sterilized Products
5.6 Clean Room Grading and Classification
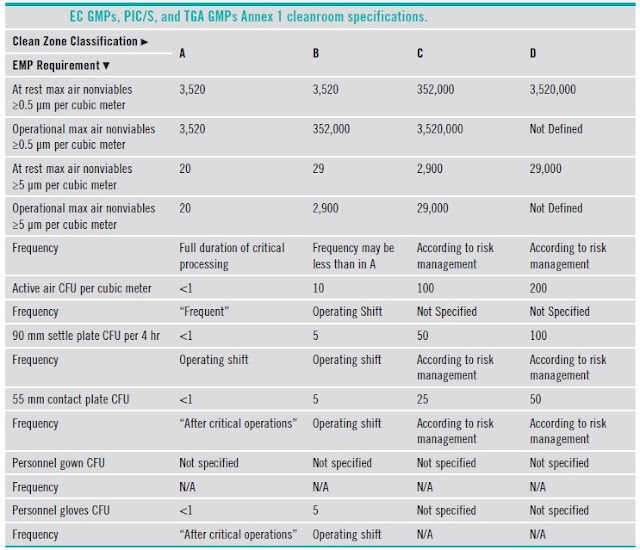
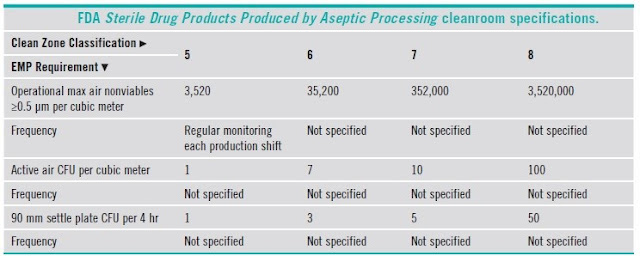
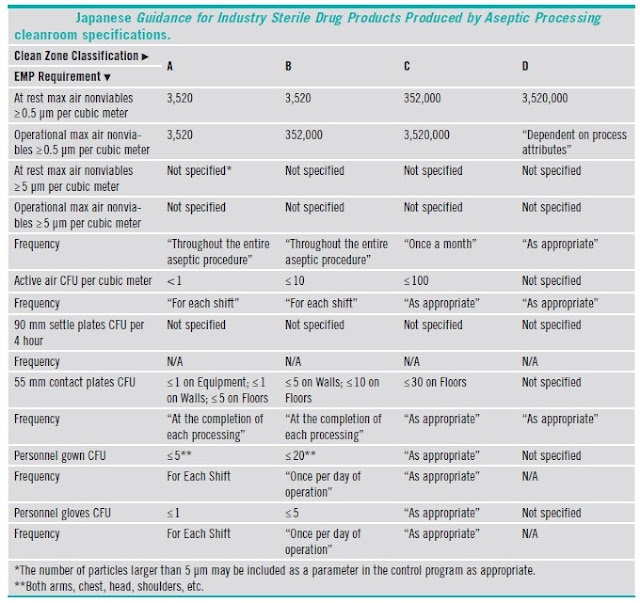
5.7 Comparison of classifications
|
WHO GMP |
US 209E |
US Customary |
ISO/TC (209) ISO 14644 |
EEC GMP |
|
Grade A |
M 3.5 |
Class 100 |
ISO 5 |
Grade A |
|
Grade B |
M 3.5 |
Class 100 |
ISO 5 |
Grade B |
|
Grade C |
M 5.5 |
Class 10,000 |
ISO 7 |
Grade C |
|
Grade D |
M 6.5 |
Class 100,000 |
ISO 8 |
Grade D |
5.8 CLEANING PROCEDURE OF STERILE AREA
5.9 PRECAUTIONS
5.9.1 Follow entry and exit procedure in critical areas. 5.9.2 Follow gowning and de-gowning procedure. 5.9.3 Moping and cleaning should unidirectional. 5.9.4 Moping should be from upper side to lower side. 5.9.5 Moping should be from machine side to door side.{getButton} $text={Download in Microsoft Office} $icon={Download} $color={Hex Color}







.webp)

%20Web%20of%20pharma%20.webp)


%20webofpharma.JPG)



