Standard Operating Procedure of Manual Optical Checking Lamp in Pharmaceuticals is describe in this post.
{getToc} $title={Table of Contents}
1 PURPOSE
1.1. It is established to provide a system for To provide the method to check the liquid inject able after filling
2 SCOPE
2.1 It is applicable to Optical checking room of Liquid parental area
3 RESPONSIBILITIES & AUTHORITIES
3.1 Supervisor
3.2 Machine Operator
3.3 Section In charge
4 REFERENCE
4.1 Quality Management Manual
5 DEFINITIONS
5.1 List of Technical Definition.
6 PROCEDURE
6.1 In sterile injectable solution, filled in either vials or ampoules product should be free from visible particulate contamination
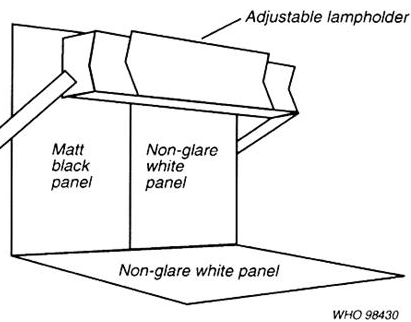
6.2 After sealing, each filled container (Ampoule or Vial) is subjected to optical inspection against white and black background. Only optically good vials/ampoules are taken for packing
6.3 Optical inspection is carried out to detect the following rejects
6.3.1 White particles
6.3.2 Black particles
6.3.3 Glass Fibers
6.3.4 Charring
6.3.5 Seal rejects
6.3.6 Mould defects
6.3.7 Low fills
6.3.8 Or any other abnormalities
6.4 Manual Inspection:
6.4.1 Take position in front of a board fixed with white and black background
6.4.2 Check the working of the fluorescent tube fixed just above the black-white plates
6.4.3 Note the batch number and product name under inspection
6.4.4 Take 3-4 ampoules and observe for sealing and molding defect
6.4.5 If sealing/molding defects are found, reject
6.4.6 Hold in front of white background plate and check for presence of black particles
6.4.7 Turn the vials upside down and inspect
6.4.8 Then check the same vials under black background and check for presence of white particles and fibers.
6.4.9 Simultaneously check for low fill also
6.4.10 If there is any doubt about the clarity of the vials or ampoules, recheck them. For any abnormality during inspection, report to the supervisor Classify the rejection appropriately and record
6.4.11 Collect good vials/ampoules in SS trays or shippers as the case may be
6.4.12 Appropriately label each tray or shipper for name of product, batch number and date of inspection
6.4.13 Confirm the correct labeling is done for all containers.
7 PRECAUTION
7.1 Always handled trained staff.
7.2 During operations avoid splashing water on optical checking lamp
8 DOCUMENTS / RECORDS
8.1 Line Clearance certificate
8.2 Identification Slip
8.3 Machinery equipment Cleaning and operational Log book
{getButton} $text={Download in Microsoft Office} $icon={Download} $color={Hex Color}




.webp)





%20Web%20of%20pharma%20.webp)


.webp)
