Standard Operating Procedure of Self Inspection in Pharmaceuticals is describe in this post.
1. Purpose:
1.1. To outline a procedure to measure the cGMP compliance to national and international standards in all departments so that overall quality management system can be maintained and improved.
2. Scope:
2.1. This SOP applies to all cGMP Self Inspection activities within Quality Assurance, Quality Control, Production, Administration, Warehouses and Maintenance participating in the Quality Management System and Good Manufacturing Practices. All the activities of Purchase and Human Resource related to cGMP are also within the scope of this SOP.
3. Responsibility:
3.1. Production Manager
3.2. QA Manager
3.3. QC Manager
3.4. QA Officer
4. Material & Equipment:
4.1. Complete manufacturing site.
5. Procedure:
5.1 AUDITOR SELECTION
5.1.1 HR Department maintains a list of trained cGMP Auditors in consultation with the Quality Assurance Manager.
5.1.2 Inspection is only conducted by Executive Director/ Managers or Personnel with appropriate experience and training as mentioned in “list of Auditors for cGMP”.
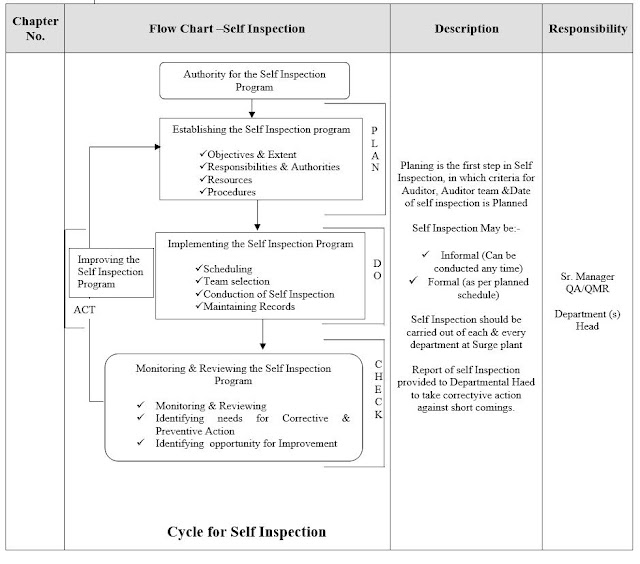
5.2 INSPECTION PLANNING
5.2.1 Annual Inspections of all departments are conducted once a year. Inspections of any or all departments may be carried out more frequently on the advice of Quality Assurance Manager or as a result of Management recommendations.
5.2.2 Quality Assurance Manager / Audit Team can also conduct Surprise Inspection of any Department without any notification to respective department and also on special occasions such as Recalls, Repeated rejections or When a GMP inspection is announced by the national drug regulatory authority.
5.2.3 Quality Assurance Manager prepares “cGMP Self Inspection Schedule” in consultation with Executive Director. Copies of Inspection schedule are circulated to all Heads of Departments (Auditee) and Auditors and take signature on the original Schedule.
5.2.4 The cGMP Self Inspection schedule is prepared on yearly basis and it contains the Inspection dates, name(s) of the nominated Auditors of internal team, time of Inspection and departments under Inspection. Minimum two Auditors (if possible) are essential in a single Inspection as per list of Auditors for cGMP.
5.2.5 Audit Teams are responsible to complete the Inspection of all the departments.
5.2.6 In case of any change (Auditor, date etc.) cGMP Self Inspection Schedule may be revised and issued by the Q.A Manager and changes are conveyed to all concerned sections / Auditors / Auditee.
5.3 INSPECTION EXECUTION
5.3.1 Before Inspection execution, team of Auditors have a general Inspection plan in mind with the following:
a. cGMP Self Inspection Check List (of department under Inspection)
b. Observation pad
c. Inspection summary Report
d. Non-Conformance Report(NC Report)
5.3.2 Auditor Team, have an opening meeting with the Auditee Manager/Section Incharge and then conduct the actual Inspection.
5.3.3 During Inspection it is verified that,
a. All the cGMP procedures related to their activities are available in the department.
b. All procedures are detailed enough and according to cGMP requirements.
c. Documented procedures and instructions are followed in their true sense and are implemented properly. The evidences i.e. quality records, for the compliance with specified procedures are checked against “cGMP Self Inspection check list”.
5.3.4 The non conformities are discussed with the Auditee in a closing session after conducting the Inspection and Non-Conformance Report forms are presented to Auditee.
5.3.5 All the activities during Inspection are noted on checklist which is submitted with Inspection report to Q.A department.
5.3.6 The Auditee (Head of Deptt./Section Incharge/Section/Function being Inspected) reviews the non conformities raised and records root cause, corrective & preventive action with target date for its completion and signs on each Non-Conformance Report form. There are four categories of findings.
a. CRITICAL
• When a main cGMP standard (national & international) is not addressed properly in department and which may results in the Hazard to Product.
b. MAJOR
• When a small part of an SOP is not being followed.
c. MINOR
• When a possibility of a non conformance in future is suspected.
d. OBSERVATION
• If there is any improvement point.
5.4 INSPECTION REPORTING
5.4.1 Non Conformities are reported on NC Report is raised.
5.4.2 For coding all Non Conformity Reports, a uniform numbering system is followed throughout the company as cGMP/00/MMYY/00.
00 = DEPARTMENT
MM = MONTH
YY = YEAR
00 = SERIAL NUMBER
Example cGMP/PDH/1212/05
5.4.3 Root Cause of non-conformance and agreed action to be taken along with responsible person and target date is mentionedNC Report.
5.4.4 Copy of Inspection Summary Report and NC Reportare provided to the Auditee while original along with filled checklist are returned to Quality Assurance Department.
5.5 FOLLOW UP
5.5.1 When actions taken are completed against a non-conformance (NC) then the NC Report form is filled with relevant remarks that are finally closed by Q.A Manager.
5.5.2 Auditor is responsible for timely follow-up of their audit NCs and closure of NCs raised.
5.5.3 All “closed” NC Report forms along with Inspection Summary Report and supporting documents are filed as Inspection Records.
5.5.4 The above closed NC Report forms and related documents are kept by Quality Assurance Deptt. as Inspection records for a retention time of two year.
5.5.5 Quality Assurance Manager updates the “Status” column of Self cGMP Inspection scheduleto monitor the inspection activities.
5.6 TARGET INPUT
5.6.1 The overall Inspection activity reports throughout the company are finally submitted to the Top Management by the Quality Assurance Manager in Management Review Meeting(MRM)for further improvements on the basis of observations and nonconformities. Nonconformities are tabulated in bar graph for each department for presenting in MRM.
5.6.2 ACCESS TO cGMP INSPECTION REPORTS
a. cGMP Self Inspection is totally an internal activity.
b. Record of Self cGMP Inspection Reports are generally for internal use to ensure continuous improvement, planning in cGMP compliance and are not subjected to external purpose/ audits.
Flow Chart 1
{getButton} $text={Download in Microsoft Office} $icon={Download} $color={Hex Color}





.webp)






%20Web%20of%20pharma%20.webp)

.webp)
